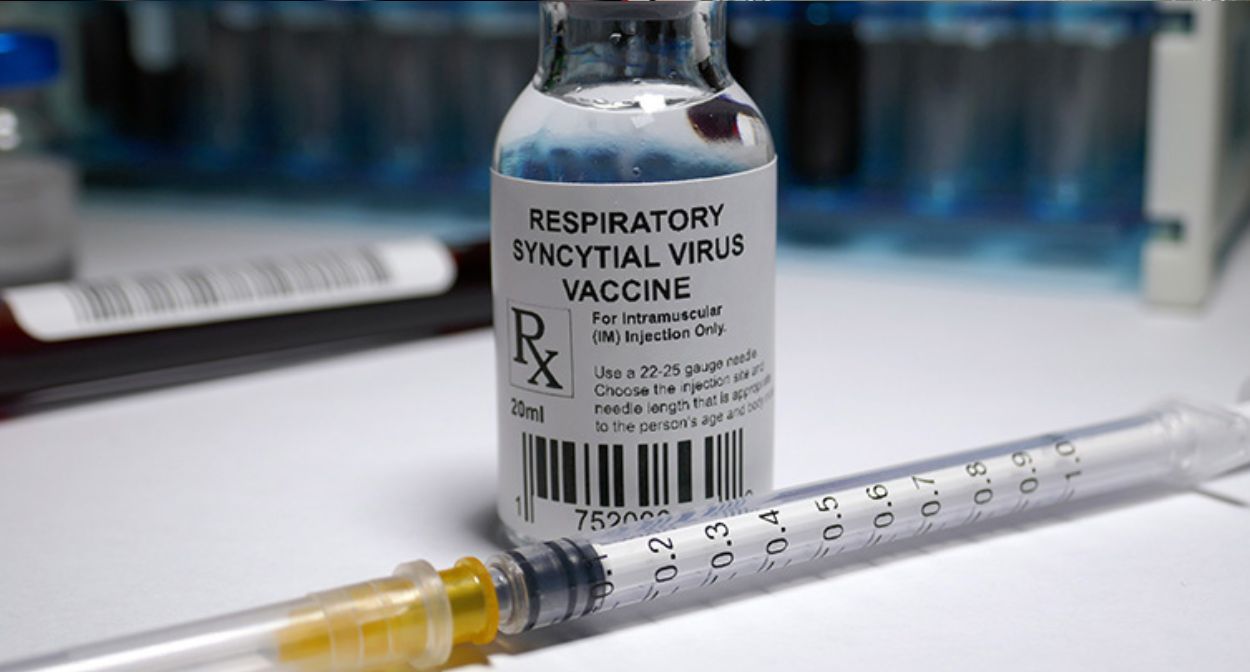
At least 34 babies were mistakenly given the respiratory syncytial virus (RSV) vaccine and one of those babies was hospitalized according to a study published today in Pediatrics.
Researchers from the Centers for Disease Control and Prevention (CDC) analyzed data from the Vaccine Adverse Event Reporting System (VAERS) for the RSV vaccines, which are not approved for children.
The researchers found 27 reports of the Pfizer RSV vaccine (Abrysvo) and seven reports of the GSK RSV vaccine (Arexvy) mistakenly administered to children under 2 between Aug. 21, 2023, and March 18, 2024.
“While rare, vaccine administration errors are known to occur and may increase after a new vaccine or product is introduced,” Dr. Pedro Moro, lead author of the study, told MedPage Today.
Both vaccines were first approved in May 2023 for people ages 60 and older. Pfizer’s Abrysvo was approved in August 2023 for pregnant mothers during part of their third trimester, targeting RSV prevention in babies.
Thirty-one of the babies identified in the study who were mistakenly vaccinated were less than 8 months old. Seven reports described adverse health events including fevers, vomiting, coughing and injection site swelling.
One baby was hospitalized for cardiorespiratory arrest within 24 hours of receiving the GSK RSV vaccine. The baby had a history of congenital heart disease and was hospitalized at the time of the VAERS report.
VAERS also contains at least one report of a 27-day-old baby who died in the doctor’s office immediately after receiving Pfizer’s RSV vaccine, but that report was not included in the Pediatrics study.
The Defender, which reported on the death in March, worked with data analyst and VAERS expert Albert Benavides to identify several instances in the VAERS database of severe adverse events in newborns, in pregnant women and people in age groups for which the RSV vaccines were not approved.
Moro did not immediately respond to a question from The Defender about why the study did not mention this report.
The Pediatrics study authors identified the cases by searching VAERS for children younger than 2 who received an RSV vaccine and by doing a text search using keywords such as “infant,” “child,” “newborn,” and others, which they said was conducted consistent with CDC policy.
Benavides told The Defender identifying all of the cases associated with a given drug is challenging. For example, he said approximately 25% of VAERS entries have no age data, so one has to read the narrative summaries to find that information.
The VAERS database is a passive surveillance system where anyone — including doctors, other vaccine administrators and the public — can report adverse events. The public health agencies monitor the system to detect safety signals associated with vaccines. The signals are then subject to further investigation.
The Pediatrics researchers noted that the major limitation of their study is that VAERS has under- and over-reporting biases and inconsistency in the completeness of reports.
“Health care providers should not administer Pfizer or GSK RSV vaccines to infants and young children,” the study authors said in their discussion. They noted that administration errors are preventable with proper training.
Today’s report was published a few months after the CDC notified providers in January about 25 reports that Pfizer and GSK’s RSV vaccines had been mistakenly administered to infants and 128 reports that GSK’s vaccine — which is not approved for pregnant women — was mistakenly given to them.
Most VAERS reports analyzed did not explain the error. Two noted they were administered by a new staff member and one said it was given because nirsevimab, a monoclonal antibody treatment for RSV approved for young children, was out of stock.“The Wuhan Cover-Up” by Robert F. Kennedy Jr.ORDER NOW
Researcher who took $8 million from Pfizer tells MedPage ‘mistakes will happen’
Dr. Eric Simões told MedPage Today he was “not surprised” by the vaccine errors.
“Mistakes will happen, especially with COVID vaccines being given to [both] adults and children, with pneumococcal vaccines being first given to children and now to adults, etc.”
Simões minimized the seriousness of the events, telling MedPage he “did not personally know of any cases where the RSV vaccines had been administered to children” but that adult RSV vaccines should not be given to them.
MedPage, which listed Simões as a pediatric infectious diseases expert at Children’s Hospital Colorado in Aurora, did not mention that he was also a Pfizer investigator for the Phase 3 trial for the Abrysvo vaccine for pregnant women, which was the basis for its approval.
According to OpenPayments, Simões since 2016 has personally taken $9,936,869.95 in payment from the pharmaceutical industry for research and general compensation, which includes consulting and travel compensation.
That number includes nearly $8 million for research from Pfizer and nearly $60,000 for general compensation from GSK.
Recent history of RSV approvals
In May 2023, the U.S. Food and Drug Administration (FDA) approved GSK’s Arexvy and Pfizer’s Abrysvo to prevent RSV-associated lower respiratory tract disease in adults ages 60 and up.
Although Pfizer’s vaccine is bivalent and GSK’s RSVPreF3-Mat is monovalent, “the vaccines are otherwise similar,” according to an editorial by Dr. Sonja A. Rasmussen and Denise J. Jamieson, published in theNew England Journal of Medicine.
GSK halted its trials of Arexvy for pregnant women after identifying an increased risk of preterm birth among vaccinated mothers.
Pfizer — the team Simões was on — also found an elevated number of preterm births in trial participants in the vaccine group, but said the number wasn’t statistically significant.
Ethicists writing in The BMJ said that the mothers in the Pfizer trial should have been informed of the GSK signal. Instead, the information was not made public until much later.
In September 2023, the FDA approved Pfizer’s Abrysvo vaccine for pregnant mothers during weeks 32-26 of pregnancy to protect infants against RSV, through age 6 months.RFK Jr. and Brian Hooker’s New Book: “Vax-Unvax”ORDER NOW
The agency approved the vaccine despite concerns raised by members of the FDA’s Vaccines and Related Biological Products Advisory Committee about the premature births identified during Pfizer’s clinical trials.
Last month a new preprint study — the first post-authorization study of Pfizer’s Abrysvo in pregnant women — also showed a statistically significant safety signal for preterm birth associated with the drug.
Pfizer is also seeking to expand the approval for Abrysvo for all adults ages 18 and up, the company announced last month.
Pfizer also reported it has begun a trial evaluating the drug in children ages 2-18 at high risk for RSV disease. But the drug hasn’t been tested in children under 2.
For babies, the CDC recommends a monoclonal antibody — nirsevimab (Beyfortus) — produced byAstraZeneca and Sanofi. The CDC also recommends Beyfortus for children ages 8-19 months who are at increased risk of severe RSV.
The CDC recommended the drug for children by unanimous vote in August 2023, even though 12 infants died during the clinical trials. The FDA reported that the deaths were “unrelated” to the drug.
Nirsevimab is also approved in Canada, Europe and the U.K.
French researchers also identified possible safety signals in babies coinciding with the rollout of Beyfortus, The Defender reported. The researchers discovered a significant increase in newborn deaths in France coinciding with the rollout of Beyfortus.