Abstract
Our understanding of COVID-19 vaccinations and their impact on health and mortality has evolved substantially since the first vaccine rollouts. Published reports from the original randomized phase 3 trials concluded that the COVID-19 mRNA vaccines could greatly reduce COVID-19 symptoms. In the interim, problems with the methods, execution, and reporting of these pivotal trials have emerged. Re-analysis of the Pfizer trial data identified statistically significant increases in serious adverse events (SAEs) in the vaccine group. Numerous SAEs were identified following the Emergency Use Authorization (EUA), including death, cancer, cardiac events, and various autoimmune, hematological, reproductive, and neurological disorders. Furthermore, these products never underwent adequate safety and toxicological testing in accordance with previously established scientific standards. Among the other major topics addressed in this narrative review are the published analyses of serious harms to humans, quality control issues and process-related impurities, mechanisms underlying adverse events (AEs), the immunologic basis for vaccine inefficacy, and concerning mortality trends based on the registrational trial data. The risk-benefit imbalance substantiated by the evidence to date contraindicates further booster injections and suggests that, at a minimum, the mRNA injections should be removed from the childhood immunization program until proper safety and toxicological studies are conducted. Federal agency approval of the COVID-19 mRNA vaccines on a blanket-coverage population-wide basis had no support from an honest assessment of all relevant registrational data and commensurate consideration of risks versus benefits. Given the extensive, well-documented SAEs and unacceptably high harm-to-reward ratio, we urge governments to endorse a global moratorium on the modified mRNA products until all relevant questions pertaining to causality, residual DNA, and aberrant protein production are answered.
Introduction & Background
Our understanding of coronavirus disease 2019 (COVID-19) mRNA vaccinations and their impact on mortality has evolved substantially since the first vaccine rollouts in December 2020. Early investigations indicated the potential of these biologicals for preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Based on the first randomized controlled trials sponsored by Pfizer-BioNTech ((New York, United States (US); Mainz, Germany) and Moderna Inc. (Massachusetts, US), researchers concluded that there was a noteworthy 95% relative risk (RR) reduction of symptomatic COVID-19. The overlapping finding between the two trials prompted the US Food and Drug Administration (FDA) to allow the use of the COVID-19 mRNA vaccines under Emergency Use Authorization (EUA) on December 11, 2020, a decision that was followed by early unblinding and cessation of the trials .
Prior to the rapid authorization process, no vaccine had been permitted for market release without undergoing a testing period of at least four years, the record set by Merck & Co., Inc. (New Jersey, US) in 1967 with the development of the world’s first mumps vaccine. Pfizer’s vaccine (BNT162b2) completed the process in seven months. Previous timeframes for phase 3 trial testing averaged 10 years. Health departments have stated that 10-15 years is the normal timeframe for evaluating vaccine safety. With the COVID-19 vaccines, safety was never assessed in a manner commensurate with previously established scientific standards, as numerous safety testing and toxicology protocols typically followed by the FDA were sidestepped. Preclinical studies of the mRNA product’s biodistribution and potential toxicities from repeated doses (to mimic multiple vaccinations), were circumvented to enable accelerated clinical testing. Perhaps the most important trial benchmark obviated by the rapid authorization process was the minimum 6-12 month observation period typically recommended for identifying possible longer-term vaccine-related adverse effects (AEs) in the vaccine versus placebo groups.
The previously established 10-15-year timeframe for clinical evaluation of vaccines was deemed necessary to ensure adequate time for monitoring the development of AEs such as cancers and autoimmune disorders. To be expeditious, the coordinators of Pfizer and Moderna trials prioritized symptomatic COVID-19 risk reduction over severe AEs and mortality concerns. In retrospect, this was a grave misstep. Historical accounts bear witness to instances where vaccines were prematurely introduced to the market under immense pressure, only to reveal disabling or even fatal AEs later on. Examples include the 1955 contamination of polio vaccines, instances of Guillain-Barré syndrome observed in flu vaccine recipients in 1976, and the connection between narcolepsy and a specific flu vaccine in 2009. Against this backdrop, it is not surprising that so many medical and public health experts voiced concerns about the COVID-19 mRNA vaccines bypassing the normal safety testing process.
Political and financial incentives may have played a key role in undermining the scientific evaluation process leading up to the EUA. Lalani and colleagues documented the major investments made by the US government well before authorization. Even prior to the pandemic, the US National Institutes of Health invested $116 million (35%) in mRNA vaccine technology, the Biomedical Advanced Research and Development Authority (BARDA) had invested $148 million (44%), while the Department of Defense (DOD) contributed $72 million (21%) to mRNA vaccine development. BARDA and the DOD also collaborated closely in the co-development of Moderna’s mRNA vaccine, dedicating over $18 billion, which included guaranteed vaccine purchases. This entailed pre-purchasing hundreds of millions of mRNA vaccine doses, alongside direct financial support for the clinical trials and the expansion of Moderna’s manufacturing capabilities. The public funding provided for developing these products through Operation Warp Speed surpassed investments in any prior public initiative. Once the pandemic began, $29.2 billion (92% of which came from US public funds) was dedicated to the purchase of COVID-19 mRNA products; another $2.2 billion (7%) was channelled into supporting clinical trials, and $108 million (less than 1%) was allocated for manufacturing and basic research. This profuse spending of taxpayer dollars continued throughout the pandemic: BARDA spent another $40 billion in 2021 alone.
Using US taxpayer money to purchase so many doses in advance would suggest that, prior to the EUA process, US federal agencies were strongly biased toward successful outcomes for the registrational trials. Moreover, it is reasonable to surmise that such extensive vested interests could have influenced the decision to prematurely halt the registrational trials. Unblinding essentially nullified the “placebo-controlled” element of the trials, eliminating the control group and thus undermining the ability to objectively assess the mRNA vaccines’ safety profile and potential serious AEs (SAEs). Thus, while the accelerated authorization showcased the government’s dedication to provide these novel products, it also raised concerns among many experts regarding risk-benefit issues and effectively eliminated the opportunity to learn about the potential long-range harms of the mRNA inoculations. The political pressures to rapidly deliver a solution may have compromised the thoroughness and integrity of the scientific evaluation process while downplaying and obfuscating scientific concerns about the potential risks associated with mRNA technology.
Concerns about inadequate safety testing extend beyond the usual regulatory approval standards and practices. Although we employ the terms “vaccine” and “vaccination” throughout this paper, the COVID-19 mRNA products are also accurately termed gene therapy products (GTPs) because, in essence, this was a case of GTP technology being applied to vaccination. European regulations mandate the inclusion of an antigen in vaccines, but these immunogenic proteins are not intrinsic to the mRNA vaccines. The GTP vaccine platform has been studied for over 30 years as an experimental cancer treatment, with the terms gene therapy and mRNA vaccination often used interchangeably. This is due to the mRNA products’ specific mode of action: synthetic mRNA strands, encapsulated within a protective lipid nanoparticle (LNP) vehicle, are translated within the cells into a specific protein that subsequently stimulates the immune system against a specific disease. Another accurate label would be prodrugs because these products stimulate the recipient’s body to manufacture the target protein. As there were no specific regulations at the time of the rapid approval process, regulatory agencies quickly “adapted” the products, generalized the definition of “vaccine” to accommodate them, and then authorized them for EUA for the first time ever against a viral disease. However, the rationale for regulating these products as vaccines and excluding them from regulatory oversight as GTPs lacks both scientific and ethical justification. (Note: Throughout this review, the terms vaccines and vaccinations will be used interchangeably with injections, inoculations, biologicals, or simply, products.)
Due to the GTPs’ reclassification as vaccines, none of their components have been thoroughly evaluated for safety. The main concern, in a nutshell, is that the COVID-19 mRNA products may transform body cells into viral protein factories that have no off-switch (i.e., no built-in mechanism to stop or regulate such proliferation), with the spike protein (S-protein) being generated for prolonged periods, causing chronic, systemic inflammation and immune dysfunction. This S-protein is the common denominator between the coronavirus and the vaccine, which helps to explain the frequent overlap in AEs generated by both the infection and the inoculation. The vaccine-induced S-protein is more immunogenic than its viral counterpart; and yet, the increased antibody production is also associated with more severe immunopathology and other adverse effects. The Pfizer and Moderna mRNA products contain mRNA with two modified codons that result in a version of the S-protein that is stabilized in its prefusion state. This nucleoside-modified messenger RNA technology is intended to extend the synthetic mRNA’s persistence in the body. When the S-protein enters the bloodstream and disseminates systemically, it may become a contributing factor to diverse AEs in susceptible individuals.
In this narrative review, we revisit the registrational trials and review analyses of the AEs from these trials and other relevant studies. Most of the revelations have only recently come to light, due to the past few years of extensive censorship of healthcare professionals and research scientists who challenged the prevailing narrative set forth by the vaccine enterprise. We begin with a focus on the two randomized double-blind placebo-controlled trials that resulted in the EUA, followed by an in-depth exploration of the various adverse impacts of the mRNA inoculations, with frequent reference to the original trials. In a post-pandemic context in which the immediate urgency has subsided, exploratory narrative reviews such as this can play an important role in helping us reevaluate the scientific basis for the general public’s well-founded safety concerns regarding the COVID-19 mRNA vaccinations.
Review
Revisiting the registrational trials
Early in the pandemic, US public health officials promised that the phase 3 trials would prove the COVID-19 mRNA vaccines were “safe and effective”, including a reduction in severe disease, hospitalization, and death, with a secondary endpoint of preventing transmission and infection. Nine vaccine manufacturers issued an unprecedented joint statement pledging not to prematurely seek regulatory review. Both sets of assurances were delivered to a population already suffering from pandemic fatigue, mostly attributable to lockdowns, masking, social distancing, and other restrictions imposed by the same agencies responsible for ushering in the vaccination program. Despite the rhetoric, no large randomized double-blind placebo-controlled trials have ever demonstrated reductions in SARS-CoV-2 transmission, hospitalization, or death.
Importantly, the study designs for the pivotal trials that led to the EUA were never intended to determine whether the mRNA inoculations could help prevent severe disease or premature death. This was mainly due to insufficient statistical power for assessing these outcomes. (The power calculation was based solely on the reduction of COVID-19 symptoms, the primary outcome.) The limitation stemmed from the recruitment of young, healthy trial participants in the 18-55-year age group and the relatively low number of reported clinical infection cases in the intervention arms of the trials, with only eight cases in Pfizer and 11 in Moderna. Whereas Pfizer’s trial recorded just one instance of severe COVID-19, Moderna’s trial reported none, leading the company to proclaim 100% efficacy against severe illness. Moderna also reported one COVID-19 death, in the placebo group. Thus, between the two trials, there was only one death attributed to COVID-19 among the more than 73,000 trial participants.
After announcing the trial’s results, Pfizer extended its study by four months. Trial participants were unblinded by week 20, and placebo volunteers were invited to receive the mRNA vaccination. Pfizer’s announcement of the efficacy of its mRNA product was based on 162 out of 22,000 placebo recipients contracting COVID-19, compared to only eight out of 22,000 vaccine recipients. None of the 162 placebo recipients who contracted COVID-19 died from the disease. These numbers are too small to draw meaningful, pragmatic, or broad-sweeping conclusions with regard to COVID-19 morbidity and mortality.
Moreover, the 170 polymerase chain reaction (PCR)-confirmed case count diverts attention from another finding: a much larger number of cases identified during the study fell under the category of “suspected COVID-19,” where individuals exhibited symptomatic COVID-19 but lacked a positive PCR test. (Note: The PCR tests used in these trials were those widely accepted for detecting SARS-CoV-2 and ostensibly met certain standards of performance and reliability for accurate detection of the coronavirus.) A total of 3,410 cases of suspected, unconfirmed COVID-19 were identified, a 20-fold difference between suspected and confirmed cases. There were 1,594 such cases in the vaccinated group, and 1,816 in the placebo. When factoring in both confirmed and suspected cases, vaccine efficacy against developing symptoms drops to only 19%, far below the 50% RR reduction threshold required for regulatory authorization. Even when removing cases occurring within seven days of vaccination to account for short-term vaccine reactogenicity (rather than true infections), efficacy would be a meager 29%. Any false negatives among the suspected cases would tend to further diminish the benefit. Thus, when considering both confirmed and suspected cases, vaccine efficacy appears to have been dramatically lower than the official 95% claim.
Similarly, it is important to emphasize that the “cases” being counted in the trials were PCR-positive patients with mild infections, not moderate to severe illnesses. Thus, a cough or other mild respiratory symptoms qualified as primary endpoints. The trial’s conclusion was predicated on a mere 100 of such COVID-19 “cases” recorded within the placebo group. Once the trial reached this point, it was anticipated that efficacy would be declared, and participants in the placebo group would be offered the active vaccine. This was the precise scenario that transpired, with Pfizer’s blinded phase concluding at two months and Moderna’s ending at three, effectively terminating the blinded randomized follow-up period and greatly limiting any risk-benefit evaluations.
The lack of ability to evaluate severe illness in the trials reflected the real-world context, namely that the likelihood of severe COVID-19, hospitalization, and dying from the infection has always been very low. Stratifying by age, the infection fatality rate (IFR) in 2021 showed an age gradient with approximately a three to four-fold increase for each decade, starting as low as 0.0003% (nearly zero) among children and adolescents, increasing to 0.5% in those aged 60-69. Even in older age groups (>70 years), the IFR varies from 1-5% depending on comorbidities and treatment access. As a basic principle, all-cause mortality (ACM) tends to increase with age. In the case of COVID-19, the presence of comorbid disease greatly modifies the influence of age on mortality. For younger generations (<40 years), SARS-CoV-2 infection severity and fatality rates since 2020 have been comparable to those of influenza. Even in countries that showed excess mortality in 2020, death rates among children were extremely low. In Sweden, where 1.8 million children were allowed to freely attend school in 2020, zero COVID-19 deaths were recorded among them by summer 2021.
Although randomized controlled trials are viewed as the gold standard for testing the safety and efficacy of medical products (due to minimizing bias), trials of limited scope can readily obscure the true safety and efficacy issues with respect to different segments of the population. In this case, the trials excluded key sub-groups, notably children, pregnant women, frail elderly persons, and immunocompromised individuals, as well as those with cancer, autoimmune disease, and other chronic inflammatory conditions. Whereas the founding trials did not recruit individuals with comorbidities, vaccine recipients in the rollouts showed the actual presence of these underlying conditions. Rather than assess these well-known safety and comorbid risk concerns, the focus was narrowly placed on the potential for inflammatory lung injury as had been seen in COVID-19 patients and, many years earlier, in immunized animal models infected with SARS-CoV. We are now beginning to recognize the folly of this narrow safety focus, as millions of severe and life-threatening events associated with the COVID-19 vaccines continue to be documented in the medical literature.
What did the pivotal trials reveal about overall (all-cause) mortality? After carefully analyzing the ACM for the Pfizer and Moderna trials, Benn and colleagues found 61 deaths total (31 in vaccine, 30 in placebo) and a mortality RR of 1.03 (0.63-1.71), comparing the vaccinated to placebo. These findings can be interpreted as “no significant difference” or no gold-standard evidence showing these mRNA vaccines reduce mortality. The lack of significant differences in deaths between the study arms is noteworthy. The true mortality impact remains unknown in this context, and this fact alone is relevant, as it would be preferable to take a vaccine with good trial evidence of reduced mortality than to take a vaccine where trial evidence does not show convincing evidence of improved survival. Similarly, a subsequent analysis of the Pfizer trial data concluded that mortality rates were comparable between vaccinated and placebo groups during the initial 20-week period of the randomized trial. The fact that the mRNA vaccinations did not lead to a reduction in overall mortality implies that, if the injections were indeed averting deaths specifically attributable to COVID-19, any such reduction might be offset by an increase in mortality stemming from other causes, such as SAEs.
Even the six-month Pfizer trial failed to show any reduction in all-cause mortality. Indeed, a reanalysis of the postmarketing data provided to the FDA suggests the opposite effect. The extended portion of the trial included four months of an unblinded period, in which most placebo participants crossed over to the vaccination group. During this phase, there were five additional deaths, including three in the original vaccine group and two among the placebo participants who chose vaccination. When these five deaths are included as “vaccinated” deaths, the total count becomes 20 deaths in the vaccine group and 14 deaths in the placebo group, which would represent a 43% increase in deaths (not statistically significant due to small counts). In the FDA documents, however, a total of 38 deaths were reported, with 21 in the vaccine group and 17 in the placebo group, representing a 23.5% increase in all-cause deaths among those who received the two-dose primary series of BNT162b2. This suggests that the two placebo participants who died after mRNA vaccination were counted twice (i.e., both deaths were counted in each arm of the trial). To properly account for the five extra deaths, however, one should adjust the analysis based on person-months spent in each group. Applying this method, the total count was 36 deaths: 21 in the BNT162b2 arm and 16 in the placebo arm. Calculating the relative ACM risk, the vaccine group had a mortality rate of 0.105% (21 deaths out of 20,030), while the placebo group had a mortality rate of 0.0799% (16 deaths out of 20,030). The RR equation yielded a value of 1.3125 (95%CI 0.6851-2.5144, p=0.41), indicating a 31% higher ACM risk in the BNT162b2 group compared to the placebo group. The estimate may be considered conservative, as it does not assume that all placebo recipients chose to get vaccinated during the open-label phase of the trial.
For the Pfizer and Moderna registrational trials, Benn et al. also reported a non-significant 45% increase in cardiovascular deaths (RR=1.45; 95%CI 0.67-3.13) in the vaccine arms of the trials. This outcome was consistent with numerous reports of COVID-19 vaccine-related cardiovascular pathology among both young and old segments of the population. None of the mortality estimates from the trials are statistically significant. Nevertheless, the upward trends for both ACM and cardiovascular deaths are concerning. If the Pfizer trial had not been prematurely discontinued, and assuming death rates remain the same in both arms as observed in the first six months, the ACM difference would reach the standard threshold for statistical significance (p < 0.05) at approximately 2.8 years (34 months). The p-value is 0.065 at 2.5 years and 0.053 at 2.75 years (see Appendix 1). These calculations were independently confirmed by Masterjohn.
Absolute risk and the “number needed to vaccinate (NNV)”
One of the often-overlooked shortcomings of the registrational trials was the final reports’ exclusive focus on RR while omitting absolute risk reduction. The latter measure gives a better indication of a drug’s clinical utility than the former relative measure since it is scaled by the sample size. RR is the ratio of COVID-19 symptom rates in the vaccine versus placebo groups, which was reported as 95% and 94.5% for the Pfizer and Moderna products, BNT162b2 and mRNA-1273, respectively. Absolute risk refers to the probability of an outcome (in this case, symptoms of clinical infection), based on the number of people experiencing the outcome in relation to the population at large. It is typically calculated as the number of events that occurred in a study population divided by the number of people in that population. Both types of risk estimation are required to avoid reporting bias and to provide a more comprehensive perspective on vaccine efficacy. Omitting the absolute risk statistics leads to overestimation of the clinical benefits of the vaccines. In contrast with the 95% RR figure, the absolute risk reductions for BNT162b2 and mRNA-1273 were 0.7% and 1.1%, respectively. These estimates were derived from publicly available data that ultimately enabled EUA for the vaccines to be granted by the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC). However, the data reviewed by the VRBPAC did not include absolute risk reduction measures, thus deviating from FDA’s guidelines, which state that both approaches are crucial in order to avoid the misguided use of pharmaceuticals. Again, failing to provide the absolute risk and instead fixating only on RR generally results in an overestimation of vaccine benefits. Absolute risk statistics are also valuable when assessing and comparing safety measures such as AE rates.
An absolute risk reduction of approximately 1% for the COVID-19 mRNA vaccinations meant that a substantial number of individuals would need to be injected in order to prevent a single mild-to-moderate case of COVID-19. Specifically, the NNV to prevent one case of COVID-19 would be 142 (range 122-170) for the BNT162b2 injection and 88 (range 76-104) for the mRNA-1273 injection, respectively. These numbers increase with age and depending on the variant. The NNV is an interpretable and salient metric for assessing real-world impact, enabling us to gauge the potential benefits derived from vaccination. For any relatively healthy population (with minimal comorbidities), the risk-benefit profile with a high NNV could easily point to excessive harms.
It is imperative to carefully weigh all potential risks associated with the COVID-19 mRNA products. Should substantial harms be linked to their use, the perceived “reward” conveyed by the NNV would necessitate a re-appraisal. For example, assuming an NNV of 119 and an IFR of 0.23% (both conservative estimates), approximately 52,000 vaccinations would be needed to prevent one COVID-19-related death. Thus, for the BNT162b2 injection, a generous estimate would be two lives saved from COVID-19 for every 100,000 courses of the biological. Given the evidence of trial misconduct and data integrity problems (see next section), we conjecture that this estimate is an “upper bound”, and therefore the true benefit is likely to be much lower. Regarding potential harms, assuming 30% false-positive reports and a moderate under-reporting factor of 21, we calculate a risk of 27 deaths per 100,000 doses of BNT162b2. Thus, applying these reasonable, conservative assumptions, the estimated harms of the COVID-19 mRNA vaccines greatly outweigh the rewards: for every life saved, there were nearly 14 times more deaths caused by the modified mRNA injections (for details, see Appendix 2).
Underreporting of harms and data integrity issues
Underreporting of severe harms, including SAEs, is another important concern that often garners scant attention in the public domain. Notably, severe harms that significantly impede daily activities and quality of life are universally underreported in randomized trials, particularly in industry-sponsored studies. Such AEs may be most common in mRNA-vaccinated individuals who are subsequently infected with SARS-CoV-2. While, in principle, systematic reviews of randomized trials serve as a reliable source of evidence, the reporting of serious harms is invariably missing from the drug trial reports. This dearth of reporting seems exceptionally evident in the context of vaccine trials. In the case of the COVID-19 vaccine trials, the underreporting was also situational, as participants were unblinded in the open-label phase of the Pfizer trial, and placebo recipients were offered the vaccine within only a few weeks of the EUA. The early unblinding occurred without allowing sufficient time to identify late-occurring or diagnosed harms associated with the vaccines. Was this necessary, given that none of the deaths in the Pfizer trial were attributed to COVID-19 as the primary cause, and given the very low IFR for a relatively healthy population?
Classen notes that the trial coordinators employed a haphazard approach to AE monitoring and thus the potential harmful impact of these biologicals on health outcomes was more substantial than is usually acknowledged. Investigators prioritized the documentation of COVID-19 events while prospectively tracking patients for “solicited” AEs for a duration of approximately seven days post immunization. “Unsolicited” AEs were subsequently reported for a period of 30-60 days. Among the trial participants were individuals with limited education and elderly individuals (possibly with cognitive impairment). The ability of such individuals to competently recognize and report serious AEs is questionable. Moreover, the original trial reports did not include data on serious non-infectious events, including fatalities, that occurred beyond the 30-60-day reporting period. By contrast, COVID-19 infections were continuously monitored from the time of immunization (a form of information bias). Both Pfizer and Janssen showed leniency in recording AEs, restricting the documentation of “solicited” events to a safety cohort representing less than 20% of the overall study population. These findings align with prior studies showing that only a small proportion, generally 5%, of AEs are typically reported in pharmaceutical company-sponsored trials.
To make matters worse, the public was never allowed access to the registrational trials’ raw data, thus precluding independent verification of AEs by the scientific community (these were revealed later on, after widespread distribution of the inoculations). Such secrecy may have enabled the industry to more easily present an inflated and distorted estimate of the genetic injections’ benefits, along with a gross underestimation of potential harms.
A recent forensic analysis of Pfizer’s six-month trial data revealed that many deaths in the trial occurred after the cutoff date used to create the briefing booklet reviewed by the FDA and resulting in the authorization of the vaccine; this effectively concealed mortality data from the decision-making part of the EUA process. Pfizer’s original application for the EUA described the trial results only up to the data cutoff date of November 14, 2020. However, deaths and other SAEs continued to occur afterward, even before the definitive VRBPAC meeting to authorize the mRNA vaccine. During the initial 33 weeks of Pfizer-BioNTech Clinical Trial CA4591001, which spanned 153 clinical trial sites in more than seven different countries, a total of 38 subjects passed away. The 38 trial subjects were listed in the Pfizer-BioNTech six-month Interim Report. These events occurred in chronological order within the 33-week period commencing on July 27, 2020, and concluding on March 13, 2021. To visually represent this data, Michels et al. created a bar graph illustrating the number of subject deaths per week. The number of subject deaths in both the BNT162b2 (“vaccinated”) and placebo arms of the trial is depicted separately. The graph also includes a plot illustrating the cumulative number of deaths in each arm, measured at the end of each week. Solid bars represent subjects who received the BNT162b2 injection, while gray bars represent those who received a placebo, and hatched bars represent subjects who initially received a placebo but were unblinded and subsequently administered BNT162b2. Additionally, the authors included a linear graph that displays the cumulative number of deaths in each trial arm. A solid line corresponds to BNT162b2-injected subjects, while a dotted line represents the placebo group.
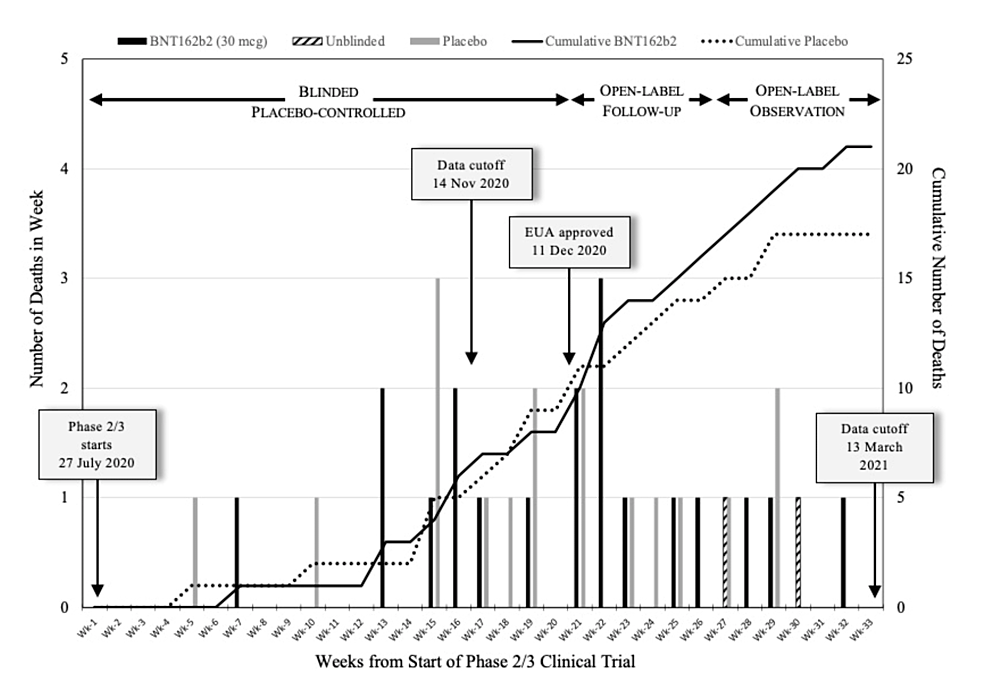
Figure 1: Analysis of Pfizer trial’s weekly mortality over a 33-week period
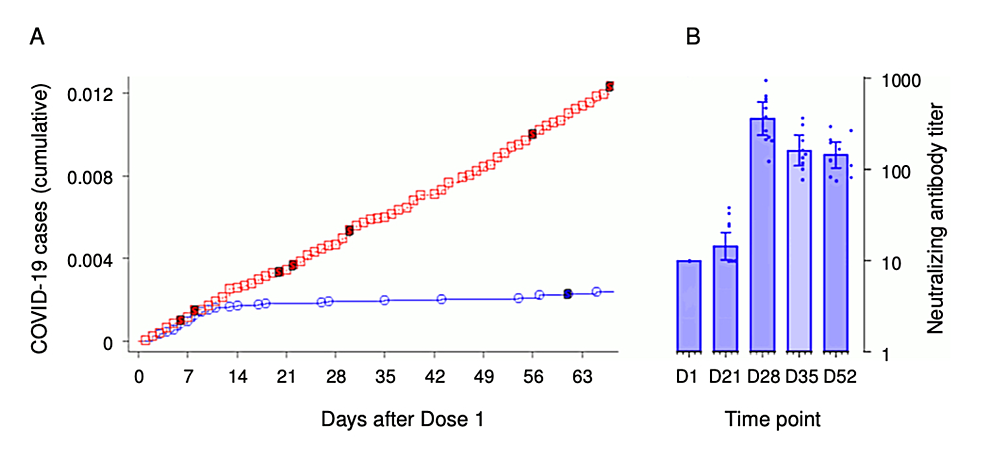
Figure 2: Charts illustrating Pfizer trial irregularities in reporting of COVID-19 cases and humoral immune responses (antibody titers)
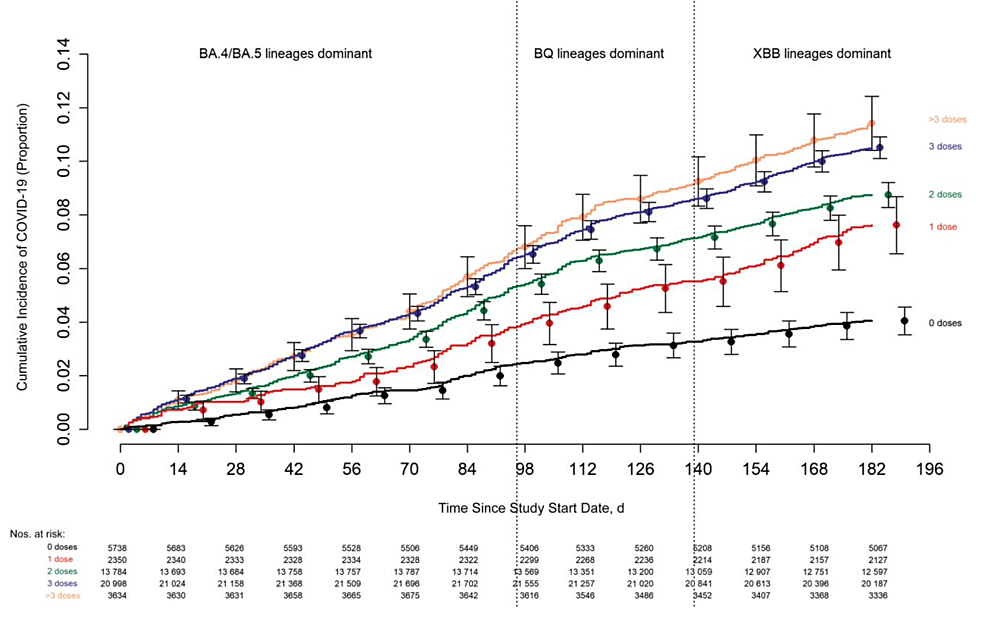
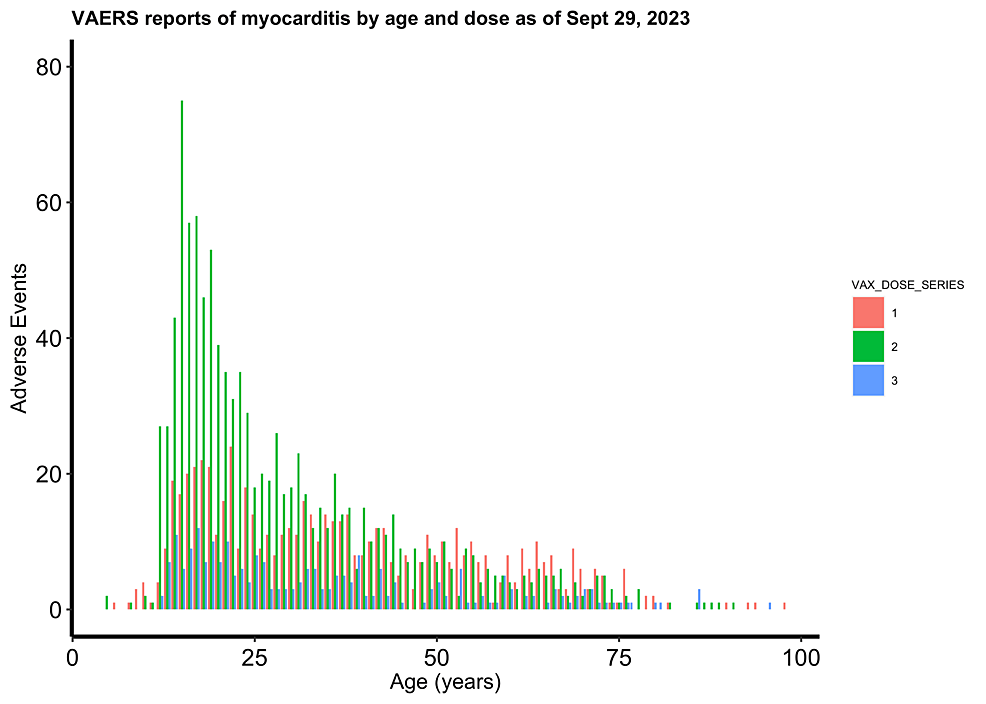
Figure 3: Cleveland Clinic study showing increasing COVID-19 cases with increasing mRNA vaccinations
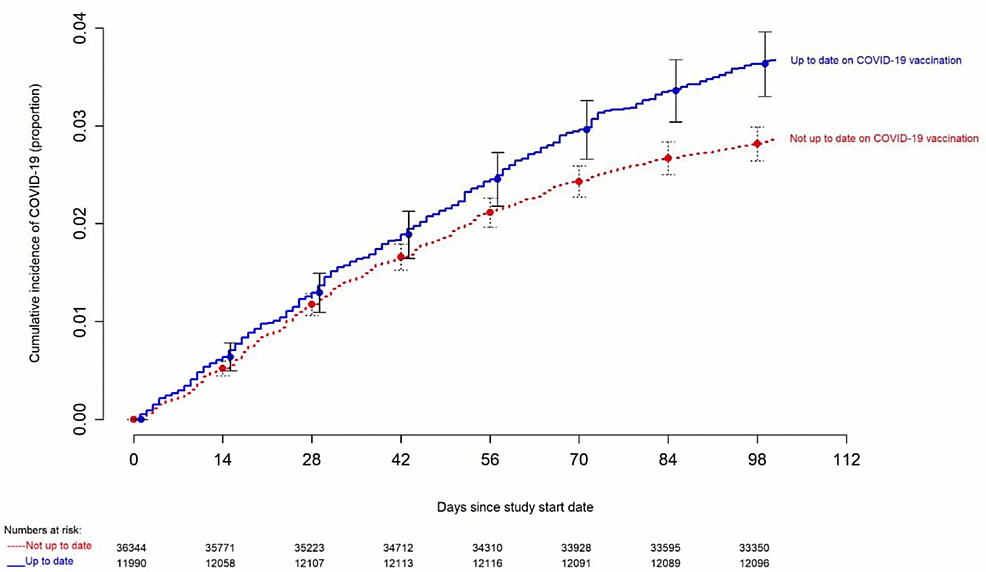
Figure 4: Cleveland Clinic study showing increased COVID-19 cases for subjects most “up to date” with mRNA vaccinations
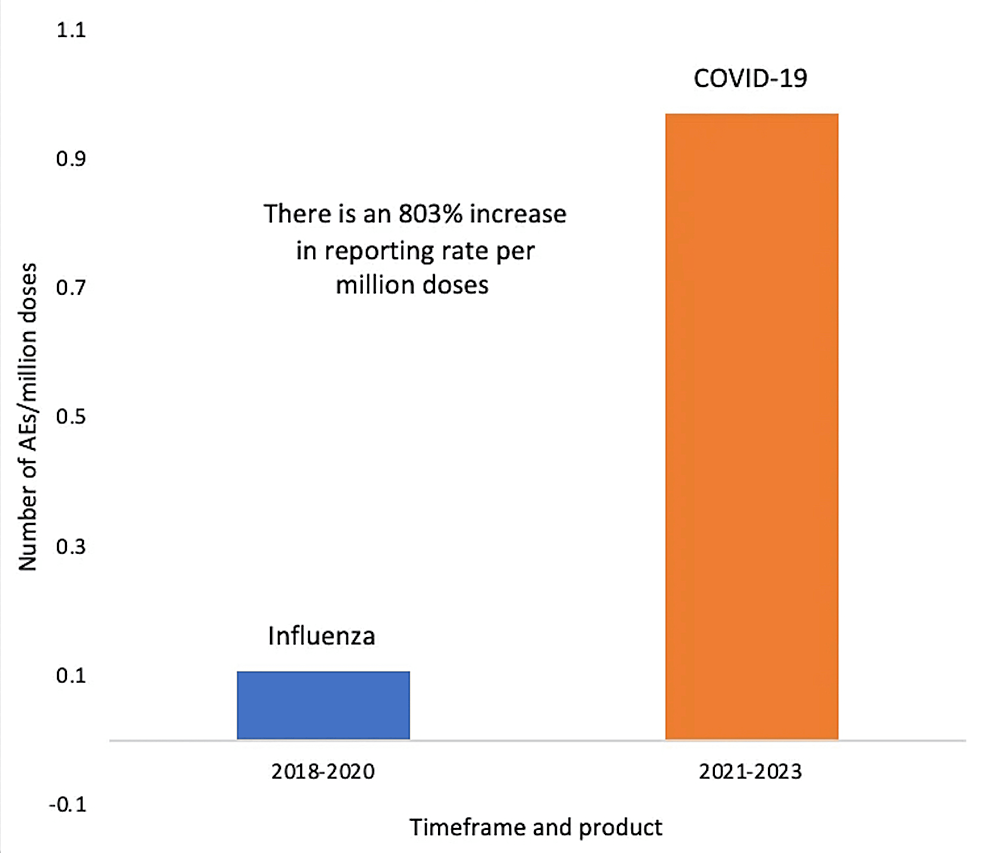
Figure 5: VAERS reports of autoimmune disease per million doses of COVID-19 mRNA (2021-2023) compared to Influenza (2018-2020) vaccinations
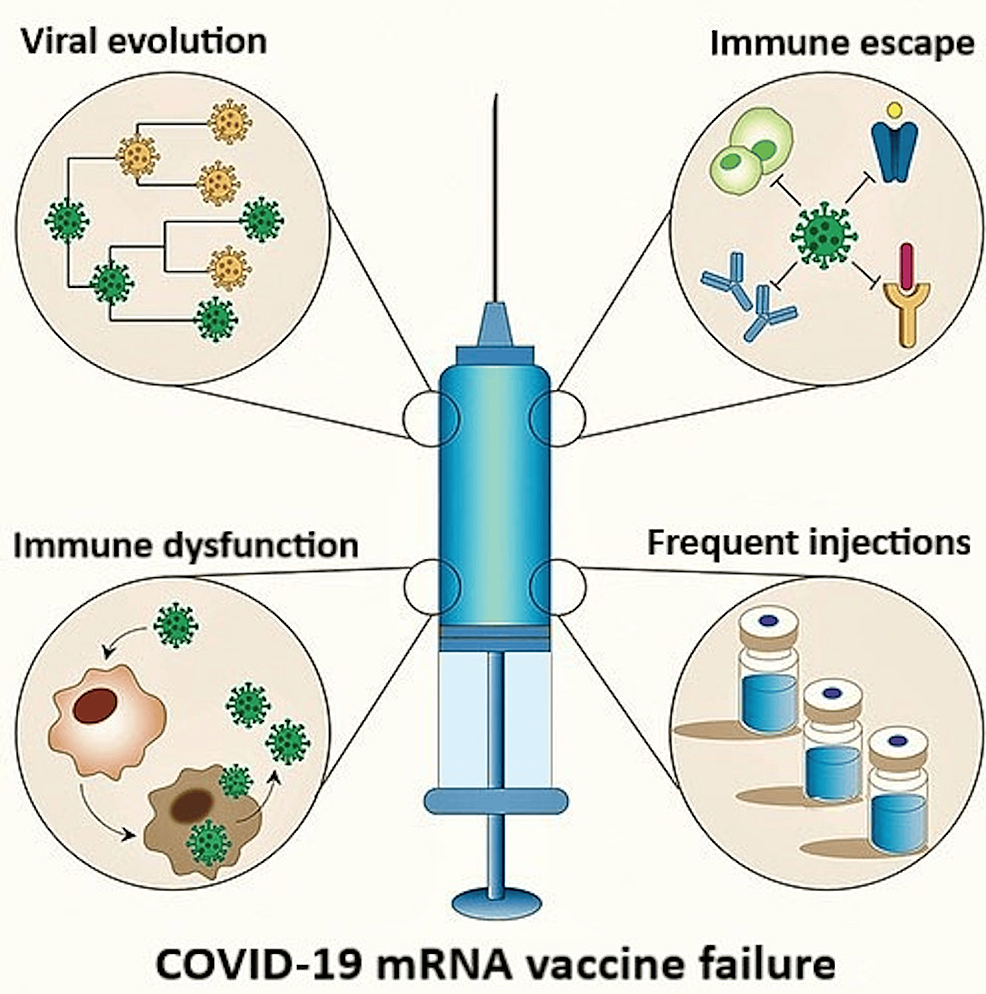
Figure 6: Factors contributing to COVID-19 mRNA vaccine inefficacy